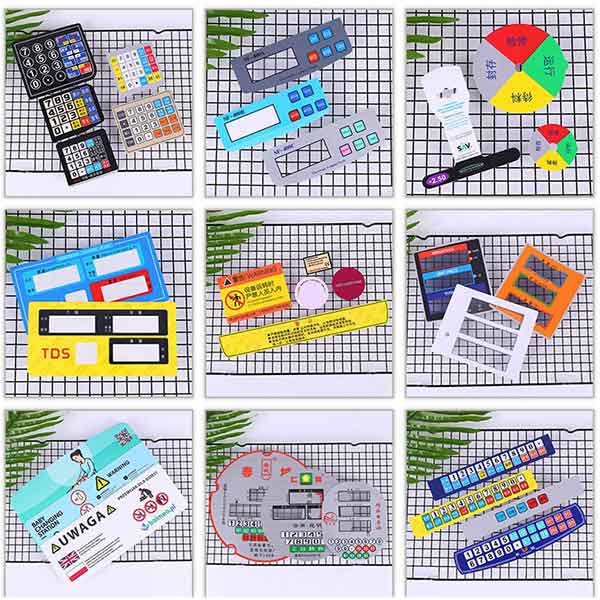
Description
Material: The most commonly used materials usually Polycarbonate and Polyester (High chemical resistance and good heat resistance)
Printing: Custom printing by silk screen printing, UV printing, digital printing, etc
Surface: Glossy, matte, texture, hard-coated
Embossing: Pillow embossing or ridge embossing
Doming: Selective doming
Windows: clear transparent windows, tinted windows, printed windows
Adhesive: whole backside adhesives or customized selective adhesives
Die cutting: laser die cutting, kiss die cutting, CNC die cutting
Ultraviolet (UV) coated for outside or not
Order FAQ
Q: What about the design file:
We can help you design a custom overlay as your application, and work with your engineering drawings to ensure a custom fit.
Vector-based files ( Adobe Illustrator, Coreldraw, PDF)
Engineering files (AutoCAD, PRO-E, STP)
Raster-based files (Adobe Photoshop, Hi-Res JPEG, TIFF)
Q: What is the necessary information to get the exact specific quotation:
| Dimension |
Please inform |
| Quantity |
Please inform |
| Material |
Please inform |
| Surface |
Glossy or matte |
| Rear Adhesive |
3M 55236, 3M9448, 3M467, 3M468, 3M9448A or as customers requests |
| Thickness |
Please inform us of the thickness you would like and the graphic overlay’s application |
| Color |
Pantone number, how many colors for printing |
| Embossing type |
embossed or flat |
| window |
LED OR LCD window |
Q: Do you have a price list?
A: There is no price list. As mostly graphic Overlays are customized printed, the material, measures, thickness, printing colors, adhesives, and windows should be different. So the Price is based on Graphic Overlay specifications and would be different also.
Welcome, contact us with your inquiry specification to get a free quote with an honest factory price.
Q: Can you produce according to the samples?
A: Yes, we can produce by your samples or technical drawings.
Q: What is your sample policy?
A: We can supply the free sample of our previous orders to you, but the customers have to pay the courier cost.
For customized samples, the sample charge would be paid before production.
Q: How to order:
A: 1. Contact us with your specifications and requests to get one exact and free quote for free, our email is info@graphicoverlaysz.com
2. Supply artwork, place the order, arrange the payment
3. Receive the approval proof and expected delivery time
4. Inform the exact address with the zip code and confirm the delivery invoice and Packing list
5. Delivery with the tracking number by express or bill number by air & sea
6. Receipt and after-sales customer service.