Medical labels are an essential component of the healthcare sector in the form of an essential element in ensuring safe and effective product usage. They are not simply identifiers; they fall under high-level regulatory controls and are designed to enhance performance with emerging technologies like smart labels, RFID tracking, and anti-counterfeiting. Since medical centers are constantly adding innovative technologies to their operations, the role of medical labels has multiplied significantly. In this article, we will discuss the essential aspects of medical labels, including their purposeful requirements, available technologies, certifications, and best practices for making them. We will also discuss the benefits of custom medical labels and share some tips for creating successful medical labels that meet the particular requirements of healthcare institutions.
1. What are Medical Labels?
Medical labels are uniquely designed identifiers that record important information regarding medical treatment, protocols, and products. Medical labels are convenient tools for clinicians, providing them with varying processes, from patient care to equipment maintenance. Labels are presented as visual prompts or reminders of processes and complement vocally announced procedure, having important information at hand and clearly understandable at any given time.
Most likely the most common application of medical labels is prescription labeling. They must contain key data points to ensure appropriate medication administration. Pharmacy labels, for instance, track intravenous (IV), oral, tablet, and compounded medication when it is being dispensed to a patient. They are also very important in replenishing automated dispensers such as Pyxis and Omnicell stations employed in hospitals for dispensing medications.
With hospital pharmacy services now starting to reach the outpatient level, the requirement for compliant and standardized pharmacy labels is more and more needed. A successful pharmacy label is crucial to both patient safety and regulatory compliance. The labels must be very specifically compliant in order to prevent medication administration errors, which would be catastrophic to patient health.
2. Key Requirements for Medical Labels
Medical labels must meet a variety of stringent requirements to ensure they effectively protect, inform, and secure medical products. These labels are not design oriented; they must serve a purposeful role by providing healthcare practitioners with information needed for the effective and safe delivery of treatments. Some of the necessary requirements that must be fulfilled by medical labels include the following:
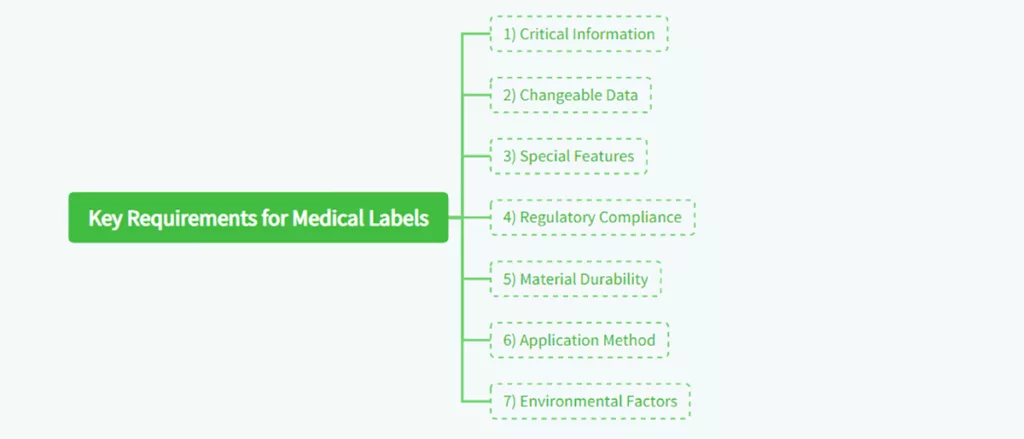
1) Critical Information
Medical marks should have necessary product information, such as the medicine’s name, directions for dosage, batch number, expiration date, and precautions and warnings, if any. The information should be clearly readable and visible even in adverse environments like operating theaters or emergency wards.
2) Variable Data
Several medical labels need space to accommodate variable data such as batch numbers and expiration dates. This is most applicable to products with a limited shelf life, i.e., drug and vaccine products. Print variable data on the labels and the healthcare community enjoys access to up-to-date information.
3) Special Features
Some of the medical labels incorporated added functions to serve the needs of healthcare personnel more effectively. An example includes using removable components to be utilized in medical records, embedded hangers that can be used to suspend infusion bottles, and multi-level labels that include extensive text written in many different languages. The additional features improve the utility and applicability of the labels within a health-related environment.
4) Regulation Compliance
Medical labels must meet local and global regulatory standards, e.g., as outlined by the U.S. Regulatory institutions such as the Food and Drug Administration (FDA) and the European Medicines Agency (EMA). The aim of regulatory demands is to keep medical products suitably labeled and information provided compatible with the registered purpose of the product.
5) Material Toughness
The substance of medical labels must be tough enough to withstand the conditions in which they are to be used. For example, drug labels stored in cold storage must withstand low temperatures without peeling off or becoming illegible. Similarly, sterilized product labels must withstand high temperatures and chemical contact without weakening.
6) Application Method
Producers need to take into account how the label is to be applied to the product—either by machine or manually. The method of application can influence the adhesive and the overall label design. For instance, machine-applied labels might require additional rigidity to ensure correct alignment, whereas manually applied labels might require a level of flexibility to allow for human susceptibility to error.
7) Environmental Conditions
Labels used in medicine need to be able to endure different environmental conditions, e.g., exposure to water, chemicals, and heat. For instance, drug labels kept in fridges or freezers should endure low temperatures without peeling or becoming stiff. The same applies to labels on equipment used in sterile environments that should endure exposure to sterilization processes, i.e., autoclaving.
3. Smart Labeling Emerges in Healthcare
With healthcare organizations trying to streamline processes and improve patient care, smart labeling technologies are increasingly in favor among most of them. Smart labels, with their track-and-trace functionality, temperature tracking and anti-counterfeiting, offer a number of benefits to healthcare organizations. Smart labels enable clinicians to have more patient time since smart labels perform activities such as inventory and product tracking automatically.
Most significant of the smart labeling technologies is implementing **Radio-Frequency Identification (RFID)** technology. Hospital beds, RFID-tagged, can track in real-time medical supplies, and these deliver valuable location data, utilization data, and date of expiration of devices and drugs. The technology reduces wastage, theft, and withdrawal of expired products to the market prior to use.
Aside from streamlining inventory control, intelligent labels are able to streamline patient safety. For example, temperature-sensitive medicines such as vaccines can have labels monitor temperature changes during storage and shipment. The label can alert medical personnel in case of increasing temperatures beyond a certain threshold so that such drugs are not dispensed to patients after they have been compromised.
Smart labeling technology is also being used to combat the growing menace of spurious drugs. Through incorporation of anti-counterfeit features such as holograms or serial numbers, smart labels make it difficult for counterfeiters to replicate real products. Patient safety is hence being protected against potentially dangerous spurious drugs, and healthcare personnel are using genuine products.
4. Label Permanence Requirements
Permanence is a necessary feature of medical labels, especially in life-or-death applications where the label has to remain intact and legible for the entire life of the product. Medical labels will likely contain information that is flat-out necessary for dosage, lab testing, drug cautionary statements, IV-line identification, patient identification, and recordkeeping. Any error or deterioration of label quality can have disastrous consequences.
For example, IV bag labels should remain readable when subjected to water, chemicals, and the rough handling of health care providers. Similarly, medication labels when stored in refrigeration must be able to withstand freezing conditions without degradation to their adhesion or hardness. Sometimes, labels need to survive exposure to sterilization procedures, e.g., autoclaving, that subject labels to heat and pressure.
For long-term performance, medical labels are produced from a better material that is abrasion, chemical, and water resistant. The adhesive too should be of strong quality to adhere onto varied surfaces like glass, plastic, and metal without curling or peeling off. Labels also need to be tested and certified for low leachable characteristics to ensure no harmful material is transferred from the label to the product.

5. Benefits of Custom Medical Labels
Even though general-use off-the-shelf healthcare labels are present, custom-made medical labels are crucial as they offer significant benefits making them enable health professionals to deliver accurate messages with their specific details. By having customized labels, patients will satisfy special guidelines, patient safety enhances, work flows get streamlined, and site-specific information are available.
For example, a typical 200-bed hospital uses up to 165 different labels, including stock and special prints. Special labels can be employed to distinguish between different drugs, to transmit dosing instructions, and to transmit important information to healthcare workers. Special labels can also be employed to accommodate the requirements of different departments of a hospital, e.g., pharmacy, nursing, and biomedical departments.
Prescribed medical labels are also more flexible in their design. Physicians can choose between different styles of different materials, adhesives, and finishes to make the label they require. A medication stored in cold storage, for example, will have a different adhesive than one stored at room temperature.
6. Common Applications for Medical Labels
Medical labels are used across a wide range of applications in healthcare settings. Among the most common applications are:
- Medication: Medical stickers are used to distinguish different medications, including anesthesia medication and doses. Stickers can be of different colors, stripes, and borders to allow practitioners to quickly recognize the correct medication.
- Pharmacy: Pharmacy labels carry detailed information on drugs, e.g., dosing directions, warnings, and expiration. Pharmacy labels act to provide assurances about the right dosing by clinical staff as well as patients.
- Nursing: Nursing labels are used for issuing directions to health workers, e.g., “Contact Nursing When Supply Is Under 10 Units.” These labels help in having vital supplies refilled before they run out.
- Biomedical: Biomedical markers are used to mark equipment with department, facility, and contact information. Equipment is guaranteed to be well serviced and maintained using biomedical labels.
- Laboratory: Laboratory labels are used to track specimens via consecutive numbering and barcoding systems. The labels help ensure handling, storage, and tracing of specimens throughout testing.
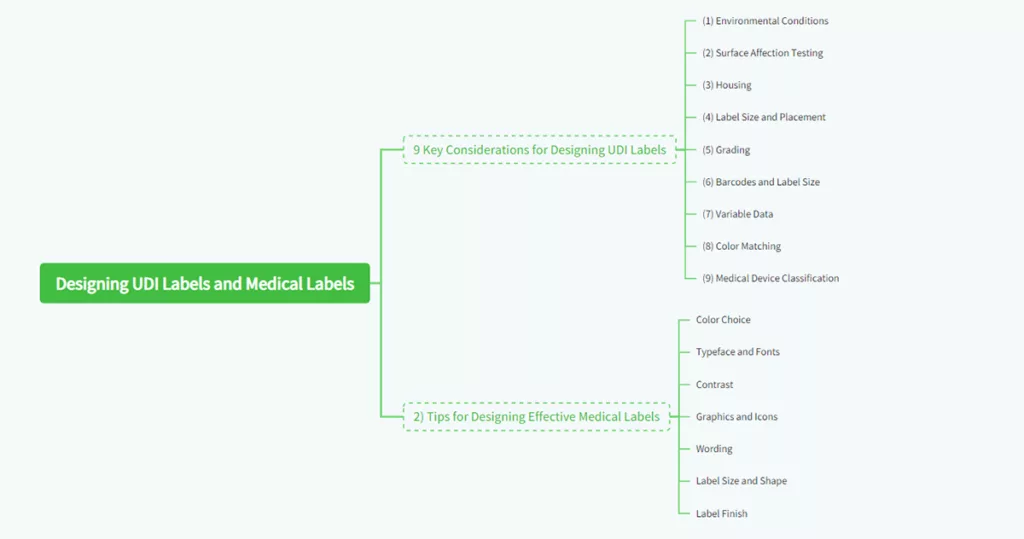
7. 9 Key Considerations for Designing UDI Labels
In designing a **Unique Device Identification (UDI)** label, there has to be adherence to the right industry best practice to ensure function and appearance. The FDA required UDI system requires marking a unique symbol on a medical device of a manufacturer which has the capability of tracking the device throughout its lifespan. The following are nine factors to consider when designing a UDI label:
(1) Environmental Conditions
Understand on what conditions your label will be run, for example, very low or high temperatures, humid places, and exposed to sunlight. You will be able to pick the most appropriate material, glue, and covering that your label should possess.
(2) Surface Affection Testing
Consider how the surface bonding material is, whether metal, plastic, smooth, or not smooth. Surface affection testing may be applied to identify any issue related to label readability or adhesion before the product is launched in the market.
(3) Housing
Consider the bonding properties of the surface material, whether it’s metal, plastic, smooth, or textured. Surface energy of material will determine the strength with which it will stick. Labels for smooth surfaces may frequently be supplied with a varying adhesive from labels for rough surfaces.
(4) Label Size and Placement
Ensure the label is big enough to cover the product and in a position that won’t get in the way of its use, e.g., when scanning for a barcode. If the label must go into a cavity or recess, then its thickness may need to be reduced so that it will be flat on the surface.
(5) Grading
UDI labels should achieve a grade of B or higher to ensure scannability. Manufacturers should provide barcode reports for record-keeping and quality assurance.
(6) Barcodes and Label Size
Ensure the label size accommodates the barcode. If space is limited, consider using a 2D barcode format, which requires less space than traditional linear barcodes.
(7) Variable Data
Ensure your label is variable data print-capable, such as serial numbers and barcodes, via digital printing capability. This is particularly required for products requiring unique identifiers to track and trace.
(8) Color Matching
Utilize Pantone color matching to ensure your label is in keeping with brand or product-specific color schemes. Periodic color matching is necessary for brand identification consistency and labels that can easily be identified.
(9) Medical Device Classification
Identify your class of medical device (Class I, II, or III) to determine the amount of control and labeling necessary. Class I devices such as bandages require little labeling, while Class III devices such as pacemakers require extensive labeling for patient safety.
8. Designing Effective Medical Labels Tips
When making your own medical labels, there are some things that you have to remember in order for the label to be able to communicate effectively. The following tips can help you create functional and appealing labels:
- Color Choice: Colors can be used to convey different feelings and levels of importance. Red, for example, means urgency, while blue means trust. Choose colors that reinforce the message you’re trying to convey and that are in line with whatever regulatory conditions are available.
- Typeface and Fonts: Use plain fonts like Arial or Helvetica that are readable, and use weight and size in combination to emphasize important information. Do not use ornamental fonts as they are difficult to read in a medical setting.
- Contrast: Use sufficient contrast between background and text to enhance readability. High contrast will enable easy reading by healthcare staff even when the label is not well illuminated.
- Graphics and Icons: Use graphics sparingly but effectively to reinforce the message. For customer-facing labels, include logos where appropriate. For medical labels that guide care, use icons that are universally understood, such as symbols for dosage or warnings.
- Wording: Apply simple action-based wording to express. Avoid using acronyms and abbreviations unless they are generally accepted. Therefore, the following should all be avoided except “as needed”:
Write out the full phrase rather than “PRN” to indicate “as needed.”. - Label Size and Shape: Choose a size and shape of a label that fit the product but hold all details required.Where space is limited, fold-out labels or multi-layered labels can be employed to offer space for longer texts.
- Label Finish: Hard finish may water, chemical, and abrasion-proof the label and therefore extend its lifespan and readability. Where the area is limited, fold-out labels or multi-layer labels may be utilized to create room for longer texts.
9. How to Choose Medical Labels?
Picking the appropriate medical label involves more than simply selecting a strong adhesive or pleasing aesthetics. In medical uses, label accuracy, legibility, and material biocompatibility have a direct impact on device performance and patient safety. The following important considerations will guide you in assessing materials, surfaces, and performance needs when choosing medical labels for your devices.
1. Environmental Analysis (Temperature, Humidity, Disinfectants, UV Exposure, and Mechanical Impact)
Medical devices are often used in harsh conditions, so environmental compatibility is one of the most critical selection factors.
Labels used in sterile rooms, hospitals, or other such environments must resist regular cleaning with alcohol, disinfectants, or chemical detergents without peeling or fading. The adhesive should be moisture-resistant and retain bond strength under humid conditions.
In low- or high-temperature applications, i.e., refrigerated storage, incubators, or autoclaves, both label material and adhesive must be stable through temperature cycles. Polyimide labels and polyester are generally preferred for these conditions due to their chemical and dimensional stability.
In outdoor or UV-exposed applications, i.e., portable diagnostic equipment, the label face must incorporate UV inhibitors or protective laminates to prevent yellowing and ink degradation.
In the event that the equipment is exposed to vibration or mechanical shock, a stronger base material with a reinforced adhesive holds the label secure throughout the product’s life.
2. Visual and Tactile Requirements (Color, Texture, Brand Identity, and Readability)
A medical label is functional as well as communicative in character. It must communicate effectively while projecting the professional image of the product.
Color contrast is required for readability, black print on a white background remains optimum for safety-critical instructions. Anti-glare coatings and high-resolution printing provide ongoing readability in the glare of operating light or fluorescent hospital lighting.
For brand identity, choose colors and finishes that harmonize with your corporate design. Clean, high-tech impressions are conveyed with smooth, glossy labels, while matte or textured finishes reduce fingerprints and improve grip. Tactile features such as raised symbols, embossed areas, or Braille also improve usability, especially in assistive medical devices.
A successful medical label needs to look professional, feel robust, and remain legible after years of use.
3. Installation and Surface Compatibility (Plastic, Metal, Coated, or Curved Surfaces)
Adhesion performance is dependent on the compatibility between the device surface and the label adhesive. Common substrates include plastics like ABS, polycarbonate, or polypropylene, and metals and painted or powder-coated surfaces. Conduct adhesion tests always under real conditions before mass production. Verify that the label does not lose its strong adhesion without curling, bubbling, or sliding after being subjected to temperature and humidity.
For curved or flexible surfaces, thin and conformable materials such as polyester films are recommended. These permit even wrapping of the label without wrinkles. For rough or textured surfaces, a high-tack adhesive allows more contact. In sterilization uses, adhesives must withstand chemical sterilants or repeated autoclaving without delamination. Proper selection of adhesive formulation for each type of surface guarantees long-term performance and patient safety.
4. Material, Thickness, Coating, and Lifespan Consideration
The combination of material, coating, and thickness determines the label’s performance in the presence of daily wear and cleaning.
- The polyester (PET) labels provide good dimensional stability, scratch resistance, and clarity and are thus suited to durable marking.
- Polypropylene (PP) is flexible and chemically resistant and hence is a suitable option for single-use devices or short-term usage.
- Polycarbonate and polyimide labels are used where high performance or thermal application is required since they possess greater strength and heat resistance.
Protective laminates or coatings can further enhance resistance to abrasion, UV light, and disinfectants. Thicker labels may be preferred for equipment surfaces that require tactile feedback or repeated handling, while thinner films are better for compact medical devices.
Lifespan testing should consider the number of cycles of cleaning or sterilization that the label can withstand without compromising adhesion or legibility. The label should be sufficiently well-designed to last and remain readable for the expected service life of the device.
5. Budget and Cost-Effectiveness Considerations
Medical labeling requires a balance between cost and performance. While more premium materials with specialty coatings offer longer-term durability, not all devices require top-of-the-line specifications. For disposable or short-term use devices, less expensive polypropylene or paper coated materials may be utilized if these can satisfy safety and regulatory standards.
For reusable medical devices, it would be a good investment to have high-performance polycarbonate or polyester labels with lower maintenance costs and fewer replacements over time.
Look not just at the initial label cost but at the overall cost of ownership, such as application, cleaning, and replacement. A little more upfront usually translates into more long-term savings in the form of reduced failures and regulatory compliance certainty.
The correct choice of a medical label involves knowledge about how environment, material, adhesion, and visual design interface. A correctly selected label resists chemicals, moisture, and wear while still offering readable identification and compliance with medical regulations. By carefully taking into account environmental conditions, tactile and visual requirements, surface compatibility, and long-term economy, you can ensure that your labeling solution will still perform throughout the life of the medical device.
10. How to Select and Buy with a Medical Labels Supplier?
Selecting the appropriate supplier for medical labels is a critical process in ensuring compliance, durability, and traceability throughout a product’s lifecycle. The healthcare sector requires labels that not only meet strict performance requirements but also satisfy regulatory compliance such as FDA, ISO 13485, or MDR. The following are key considerations in assessing suppliers and undertaking the procurement process in a seamless manner.
1. Evaluating Supplier Capability, Certification, and Experience
Technical capability and industry experience are the foundation of reliability when selecting a medical label supplier. A competent supplier will demonstrate expertise in medical-grade materials, adhesive technology, and clean manufacturing environments. They will also be knowledgeable about sterilization compatibility, biocompatibility, and traceability needs.
Look for suppliers who have ISO 13485 or ISO 9001 certification, which are indicators of compliance to quality management systems for medical device manufacturing. Additional certifications such as RoHS, REACH, or UL may also be relevant depending on product requirements.
Ask the supplier about their experience in sterilization-resistant labels, chemical-resistant coating, or UDI printing. Suppliers with experience in serving medical device manufacturers or laboratories are more likely to anticipate issues and provide compliant solutions.
Production capacity and scalability are also important considerations. Good partners can handle small prototype orders and full-scale production runs without sacrificing lead time or quality control.
2. Sample Testing and Certification Needs
Always request physical samples for testing under real-world conditions before placing orders for large volumes. Testing should include adhesion strength, chemical resistance, print durability, and readability after sterilization cycles. Conduct compatibility testing between the label material and the surface of your medical device if it is made of plastics like ABS, PC, or PP, or if it will undergo repeated cleaning.
If the product is to be exported, ensure the supplier can provide material safety data sheets (MSDS) and certificates of compliance that prove compliance with medical and environmental requirements. In some cases, a formal validation report will be required to demonstrate that the label will function in accordance with performance and safety requirements for the anticipated life of the device.
In the course of sample testing, not only material quality, but also print accuracy, barcode scanability, and pack consistency, should be assessed. Suppliers that actively support testing and certification are more likely to exhibit a stronger commitment to regulatory compliance and long-term partnership.
3. Quotation, Minimum Order Quantity, Lead Time, and After-Sales Service
In comparing quotations, do not only examine unit price, but the overall value offered. A good quotation should include details such as:
- Material type, thickness, and coating
- Printing process (screen or digital)
- Adhesive brand and performance level
- Packaging and labeling method
- Anticipated lead time and logistics options
Minimum order quantities (MOQs) need to be addressed early since the production of medical labels can have special tooling or print setups. Suppliers with flexibility and smaller batch capability for pilot runs or test phases are ideal for early-stage medical projects.
Lead time is also a serious concern. Reliable suppliers will provide you with realistic delivery dates and warn you well ahead of time of potential delays. For repeat orders, an annual forecast or supply plan can lock in pricing and production.
After-sales service cannot be ignored. A reliable supplier provides follow-up service, helps to troubleshoot adhesion or sterilization issues, and replaces defective batches. Continued contact after delivery builds trust and ensures smooth long-term collaboration.
Procuring a qualified medical label supplier entails more than a certification review, technical capability, and consistency of service review. Through testing, review of documentation, and a grasp of each supplier’s capabilities, you can be assured of continued label quality and compliance during production runs. Effective procurement is more than cost, it’s engaging in partnerships with suppliers that understand medical standards, prioritize patient safety, and deliver consistent results in the long term.
11. Regulatory and Compliance Issues for Medical Labels
Compliance is arguably the most important single aspect of medical label design and production. Medical labels are not just identification labels; they are part of the broader safety and regulatory framework that enables medical devices to be used, stored, and traced correctly throughout their life cycle. The section goes on to describe the key areas of compliance each manufacturer or supplier must know in designing or sourcing medical labels.
1) Medical Device Labeling Requirements (FDA, ISO 13485, ISO 14971, and Others)
Medical labeling is overseen by several international and regional standards that set safety, documentation, and performance requirements. In the US, the labeling of devices is regulated by the Food and Drug Administration (FDA) under 21 CFR Part 801 and 21 CFR Part 820, which govern how device information, warnings, and instructions must be presented.
FDA-compliant labels must be readable, permanent, and comprehensible under normal conditions of use and cleaning. They must also include accurate device identification, manufacturer details, and, where applicable, unique device identifiers (UDI).
On the global level, ISO 13485 sets the foundation for a medical device quality management system, including documentation control, traceability, and process validation for label production. It ensures that label manufacturing follows the same quality standards as the device itself.
ISO 14971 is focused on risk management of medical devices and requires the identification and reduction of labeling-related risks, such as misinterpretation, fading, or detachment of labels. Proper labeling prevents incorrect use or confusion, thus reducing overall product risk.
Additional standards such as IEC 60601-1 (electrical medical equipment safety) and EN ISO 15223-1 (labeling symbols and graphical requirements) may also apply depending on the device category and intended market region. Manufacturers must check which standards are mandatory for their target markets and include compliance at an early design phase.
2) Sterile Environment, Cleaning Procedures, and Compatibility with Disinfectants
The medical labels must be able to resist exposure to the process of regular cleaning, sterilization, or disinfection without breaking down and losing their readability. Both disposable and reusable devices are exposed to this requirement. The materials in a cleanroom or sterile area should not release particles or shed contaminants that would influence sterility. Low-outgassing coatings and adhesives are best to eliminate any chance of contamination.
Another key compliance factor is compatibility with disinfectants and cleaning chemicals. Labels must be resistant to alcohol, chlorine-based disinfectants, hydrogen peroxide, and quaternary ammonium compounds, which are common cleaning products in laboratories and hospitals. A label that peels, cracks, or smudges during cleaning can result in regulatory non-compliance and safety hazards.
The manufacturers need to request chemical resistance test reports or perform validation tests to ascertain that the label materials, inks, and adhesives remain stable after exposure to multiple cleaning cycles. In addition, design features such as rounded edges of labels and smooth surfaces can reduce the accumulation of contamination and improve cleanability, which supports infection control processes.
3) Traceability and Tracking Requirements
Traceability is the core of modern medical device regulation. Regulatory authorities require all medical products and their components, including labels, to be fully traceable throughout their production, distribution, and utilization.
The Unique Device Identification (UDI) system, mandated by the FDA and also the European MDR (Medical Device Regulation), gives each medical device a unique code worldwide. The code must be printed or encoded on the label in both human-readable and machine-readable formats, such as barcodes or DataMatrix symbols.
The UDI allows the regulators, healthcare providers, and manufacturers to trace the products efficiently in case of recall, adverse events, or performance tracking. To fulfill these requisites, label printing must be very precise and long-lasting, even after wear and sterilization.
For internal traceability, the suppliers are recommended to maintain batch records and quality control records that document each production run, material lot number, and delivery record. The records assist in post-market surveillance and compliance audits.
Digital traceability solutions, such as QR-coded labels or RFID-enabled tags, are also gaining traction in smart medical device supply chains. Such solutions enhance real-time visibility and support regulatory compliance across global markets.
Regulatory compliance for medical labeling is not optional; it is a critical part of product safety and quality assurance. Standards knowledge such as FDA labeling requirements, ISO 13485, and ISO 14971 ensures compliance of the labels both from a technical and legal standpoint.
While sterilization compatibility and chemical resistance considerations safeguard clinical performance, effective traceability systems facilitate global regulatory compliance, product recall management, and patient safety. By following these principles in sourcing and design decisions, manufacturers are able to achieve both operational efficiency and full regulatory confidence.
12. Medical Labels FAQs
1) What are the typical materials for medical labels?
Medical labels are typically made of synthetic films such as polyester (PET), polypropylene (PP), polycarbonate (PC), and polyimide. They are more impermeable to moisture, chemicals, and sterilization compared to paper labels. Economical coated papers can still be used for disposable or short-term devices if requirements for safety and readability are met.
2) How do I keep a label readable after it has been cleaned and sterilized?
Choose materials and inks that have been chemically and heat-resistance tested. Polyester and polyimide labels with protective laminates hold up well under repeated exposure to disinfectants, autoclaves, or UV light. Always request chemical compatibility data from your supplier and replicate cleaning cycles during product validation.
3) What is the role of adhesives in medical labeling?
Adhesives determine the manner in which a label sticks to a surface and the duration it stays there. For flat surfaces such as polycarbonate or metal panels, general-purpose acrylic adhesives usually suffice. For curved or textured surfaces, flexible high-tack adhesives are more suitable. In the medical industry, low-outgassing and solvent-free adhesives are best in order to ensure no contamination or residue after sterilization.
4) Can medical labels be printed with barcodes or QR codes?
Yes. Most medical labels nowadays carry barcodes, QR codes, or DataMatrix symbols for traceability within the Unique Device Identification (UDI) system. The print must be crisp, with high-contrast codes that will continue to scan even after being exposed to water or cleaning. Thermal transfer and UV digital print are usually used for this.
5) Must all medical labels have UDI compliance?
Not all devices require UDI, but most regulated products in the US, EU, and most other markets require it. UDI compliance is based on the device classification and intended market. Always consult local regulations, such as the FDA’s 21 CFR 801 for the US or MDR 2017/745 for the European Union, before you complete your label design.
6) How do I test the durability of a medical label?
Durability testing typically includes adhesion strength, scratch resistance, chemical resistance, and accelerated aging under UV or temperature cycling. Autoclave testing or wipe testing with hospital-grade disinfectants are also performed by some manufacturers. Testing is done to verify that the label remains intact, legible, and compliant throughout the intended service life of the device.
7) What are the most common reasons for medical device label failure?
Label failure is most often due to incorrect adhesive selection, poor surface cleaning, incompatible coatings, or insufficient curing of printed layers. Environmental stress, such as exposure to alcohol-based disinfectants or temperature fluctuations, can also cause peeling or fading. Collaborative design with an exclusive medical label supplier reduces such risks.
8) How do I know if my label supplier is in compliance with regulatory demands?
Ask your supplier for documentation of certifications such as ISO 13485, ISO 9001, or FDA registration. Request material safety data sheets (MSDS) and testing reports that confirm compliance with RoHS, REACH, and other safety standards. Reputable suppliers can also provide validation samples and traceability documentation for each production batch.
10) What maintenance practices help extend the life of medical labels?
Clean labels softly with a soft, lint-free cloth and mild detergent. Avoid using strong solvents, sharp instruments, or abrasive cleansers that can damage the surface or printed text. Regularly check for edge lift, fading, or distortion, especially on reusable medical devices. Replacement of damaged labels in a timely manner maintains compliance and patient safety.

13. Conclusion
Medical labels have a very important role in the health care industry as they provide critical information that protects patient safety, product integrity, and regulatory compliance. Regardless of how tailored label designs or advanced intelligent labeling technologies are, medical labels remain at the heart of health care processes today. Despite best practice in label design, material sourcing, and regulatory compliance, health care organizations could make their labels the most dependable, usable, and secure.
In a profession where one mistake could spell life or death, the importance of high-quality, professional medical labels really cannot be overstated. As health care centers proceed with adding new procedures and technology to their services, their demand for high-quality medical labels will just grow. With investments in custom medical labels and smart labeling technologies, health care professionals are able to streamline the efficiency of their processes, reduce wastage, and improve patient care.





Leave A Comment